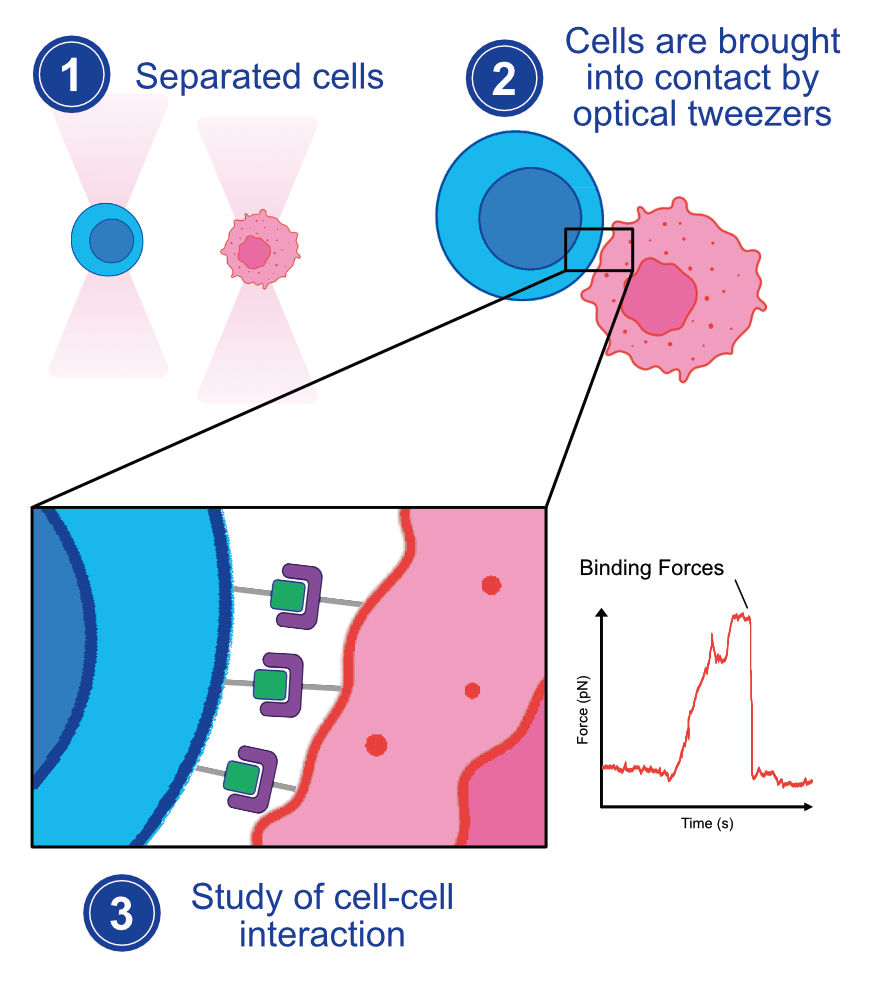
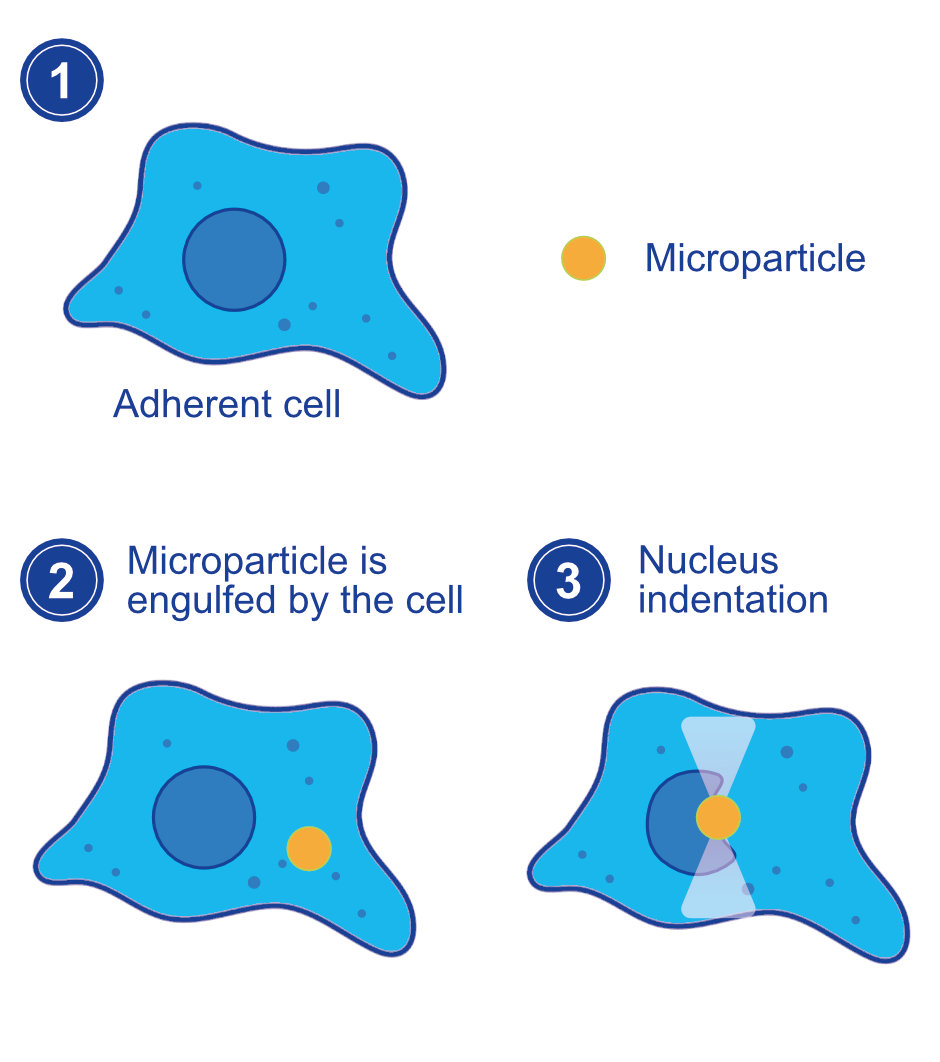
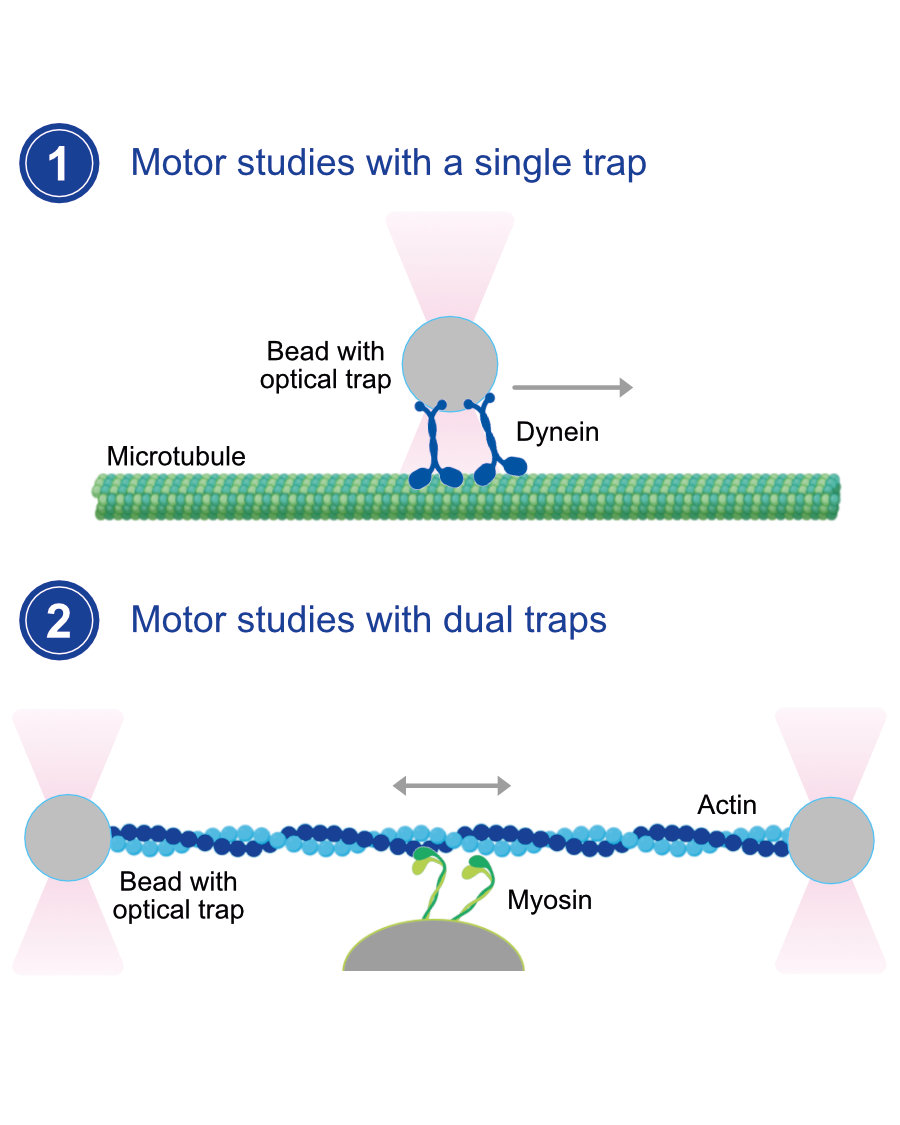
Optical Tweezers: Empowering Precision at the Nanoscale
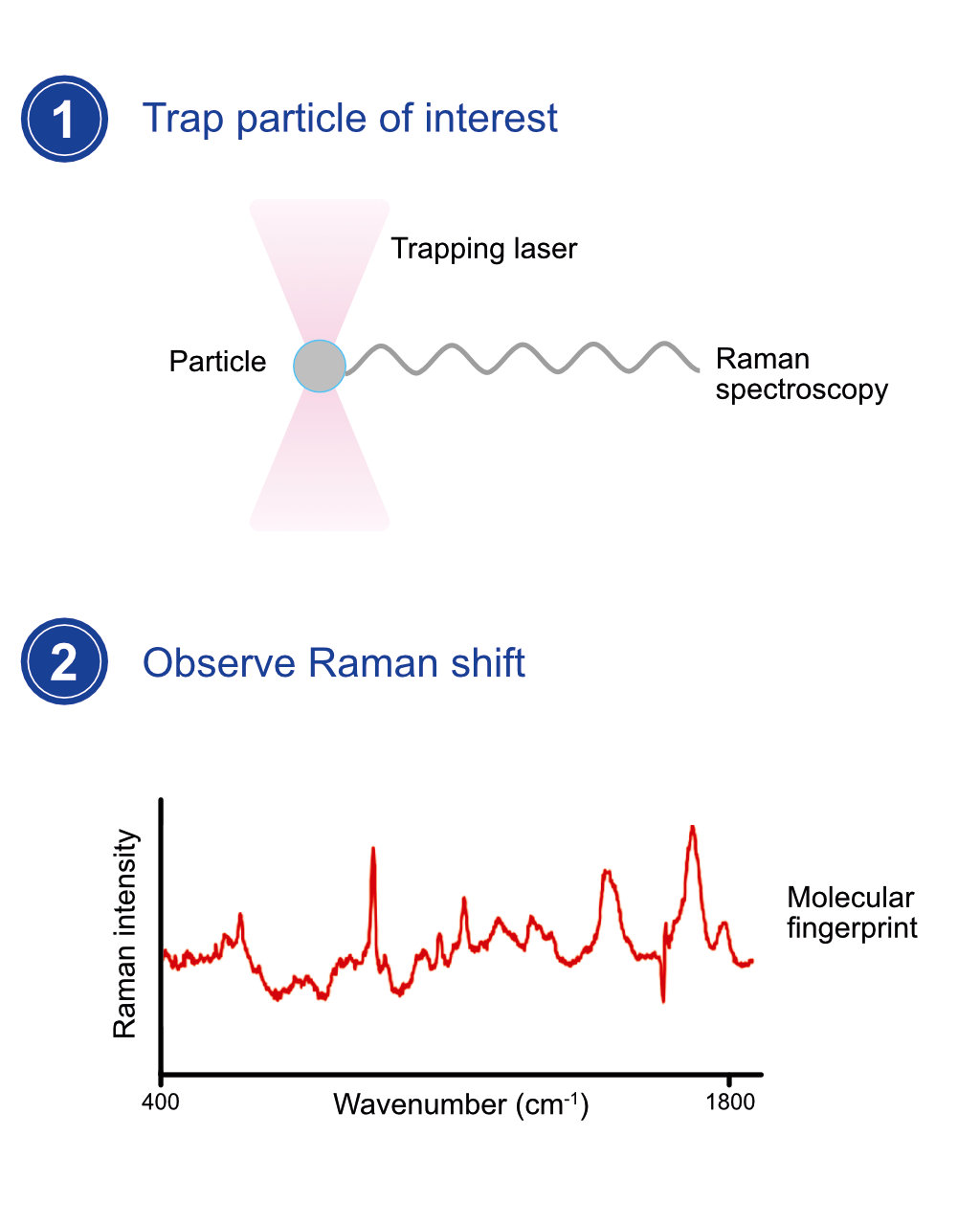
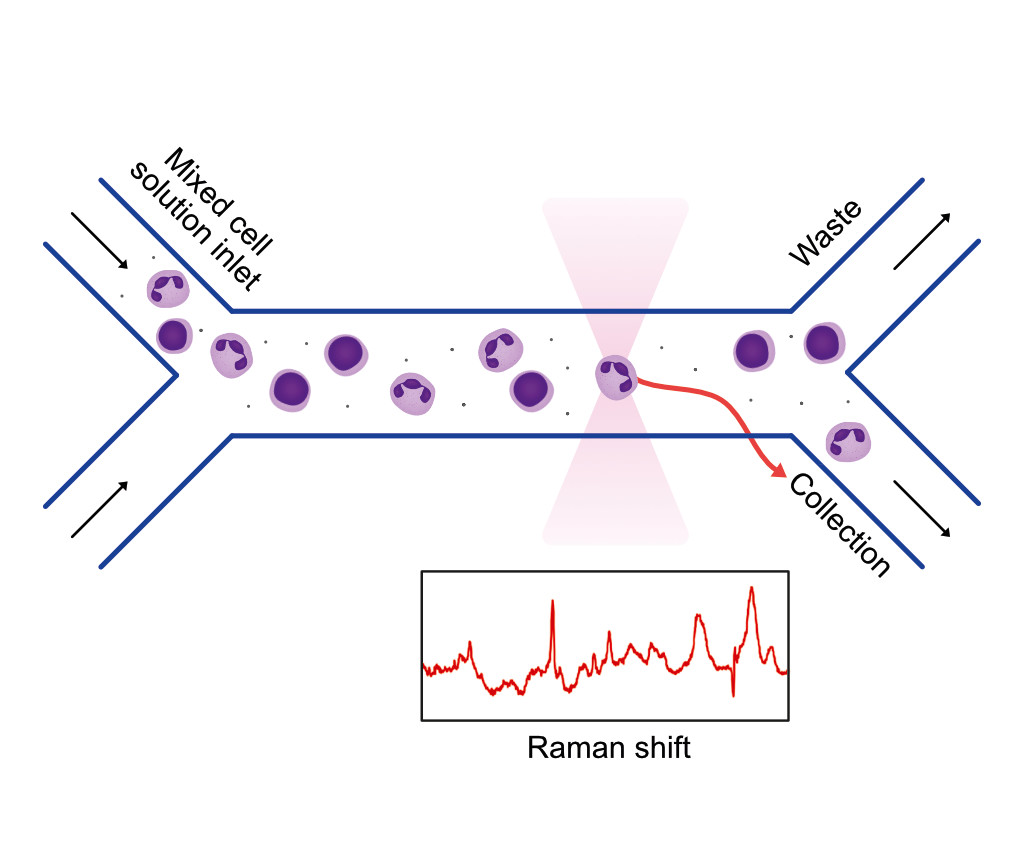
The MMI optical tweezers system is based on the mechanical forces arising from a strongly focused laser beam. It enables comfortable, ultra-precise and contact-free manipulation of up to 20 microscopic particles, living suspended cells, or subcellular organisms and the measurement of intracellular activities. The new generation of CellManipulator 3.0 offers all functionalities of the previous optical tweezers system with efficient yet compact electronics that benefit from an embedded system and microprocessor.
How can the MMI optical tweezers be applied to your research?
Do you want to make novel discoveries in life science? Do you need a non-invasive technique to spatiotemporally control the movement or the interaction of the nano/micro-metric-sized objects and to study them? The MMI optical trapping solution is the answer. Independent of the application/topic we will find the right solution for you.

This is what our customers appreciate
The MMI CellManipulator optical tweezers was customized on an upright microscope upon our request. The optical tweezers has been working reliably, with excellent manipulation power and flexibility on various devices, from simply glass slides to microelectrodes on silicon. The MMI service was also professional, fast and considerate.
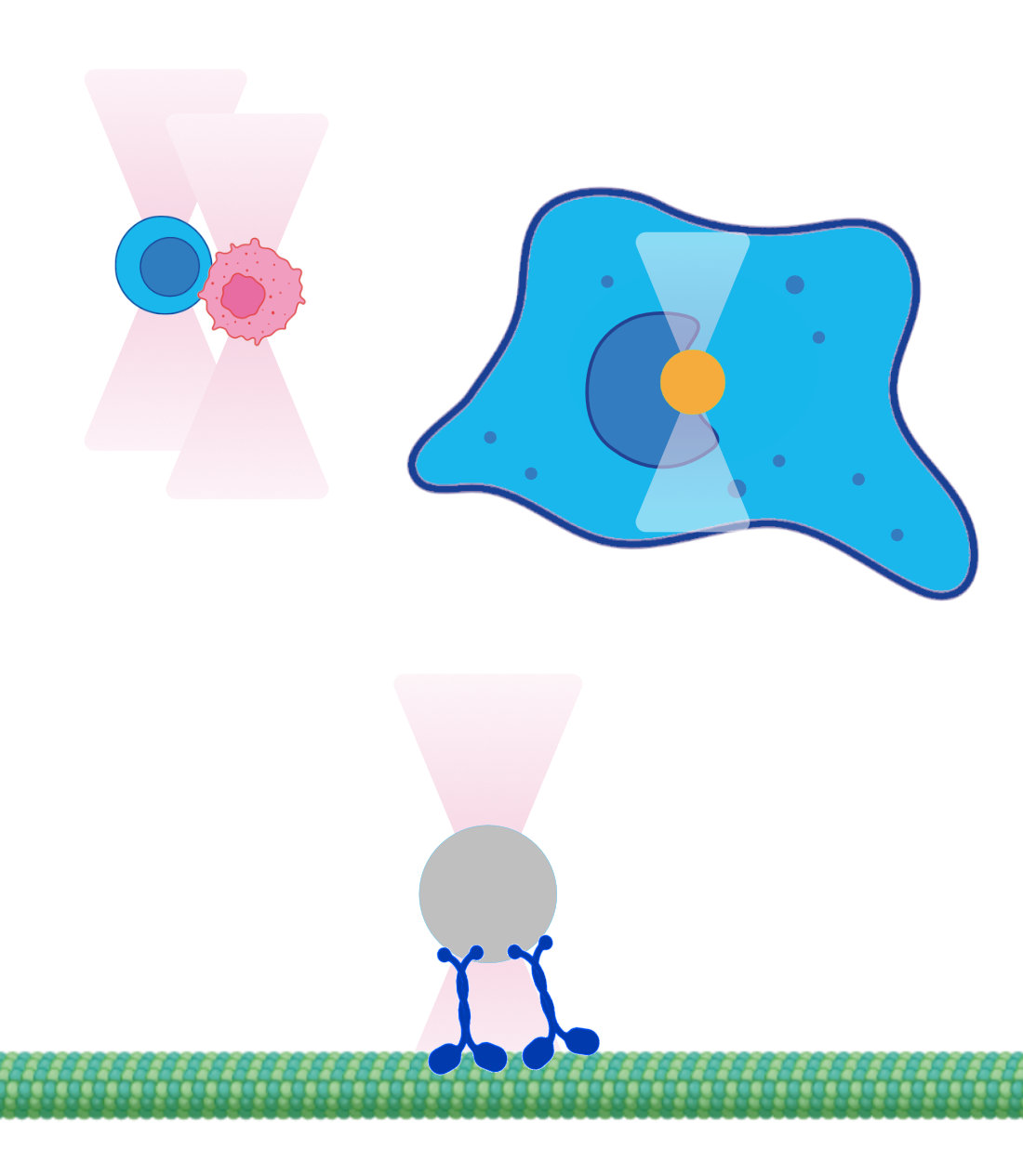
Optical trapping of living organisms with the MMI optical tweezers
Trapping swimming bacterium
Detaching bacterium
Trapping diatom
What are Optical Tweezers?
In this video, the optical trapping theory as well as optical tweezers are explained and the video also visualizes how laser traps are generated and how laser tweezers work:
Are there any limitations of optical tweezers that I need to be aware of?
Cell Mol Biol. – 1998
Time-gated autofluorescence microscopy of motile green microalga in an optical trap.
Koenig et al.
Review of Scientific Instruments. – 2007
Andersson et al.
Nature protocols. – 2007
Construction and calibration of an optical trap on a fluorescence optical microscope.
Lee et al.
Traffic. – 2009
Sparkes et al.
Current Opinion in Plant Biology. – 2010
Optical tweezers for the micromanipulation of plant cytoplasm and organelles.
Hawes et al.
New Phytologist. – 2010
Actin and myosin regulate cytoplasm stiffness in plant cells: a study using optical tweezers.
van der Honing et al.
Nano LIFE. – 2012
The biomechanics of drug-treated leukemia cells investigated using optical tweezers.
Zhou et al.
IET Nanobiotechnology. – 2012
Hepatitis B surface antigen–antibody interactions studied by optical tweezers.
Zhou et al.
Journal of Cellular Physiology. – 2013
Frequency-dependent cell death by optical tweezers manipulation.
Ng et al.
Journal of Modern Physics. – 2014
The laser technology: new trends in biology and medicine.
Legres et al.
2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. – 2014
Mechanical characterization of ART-treated Jurkat cells using optical tweezers.
Khakshour et al.
RSC Advance. – 2014
Zhou et al.
ACS Publications. – 2014
Ceragenin Mediated Selectivity of Antimicrobial Silver Nanoparticles.
Hoppens et al.
PLOS ONE. – 2015
Khakshour et al.
Scientific Reports. – 2016
Mechanical oscillations enhance gene delivery into suspended cells.
Zhou et al.
Scientific Reports. – 2016
MINDEC-An Enhanced Negative Depletion Strategy for Circulating Tumour Cell Enrichment.
Lapin et al.
ACS Nano. – 2016
Coupling of Retrograde Flow to Force Production During Malaria Parasite Migration.
Quadt et al.
Microscopy Research and Technique. – 2016
Multimodal and Non-Linear Optical Microscopy Applications in Reproductive Biology.
Adur et al.